Overview
ALLERSPRAY-G offers a novel approach to managing allergic rhinitis, providing a mechanical shield against environmental allergens. This Class IIa medical device is designed to alleviate symptoms associated with allergic rhinitis, including nasal congestion, rhinorrhea, sneezing, and itching, without the use of medication.
Innovative Formulation
ALLERSPRAY-G uniquely combines glycerol, solagum, tannin-rich plant extracts, and food-grade preservatives to form an osmotic, stable, and non-irritating barrier. This formulation not only protects but also hydrates the nasal mucosa, offering immediate comfort and long-term relief.
Mode of Action
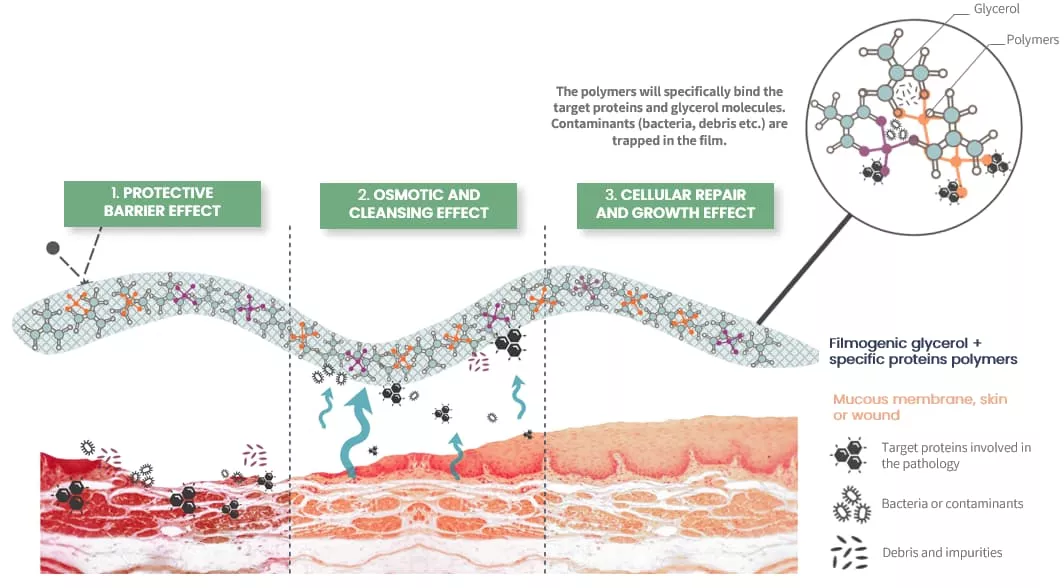
- Protective Barrier: Establishes a filmogenic shield on the nasal mucosa, preventing irritants from triggering allergic reactions.
- Mechanical Cleaning: Utilizes osmotic activity to remove contaminants, reducing inflammation and symptoms.
- Cellular Repair: Promotes healing and regeneration of the nasal mucosa for sustained relief.
Key Benefits
- Significantly reduces both nasal and ocular symptoms of allergic rhinitis.
- Diminishes the need for additional medication.
- Offers a dual-action solution: relief and protection.
- Improves overall quality of life without causing drowsiness.
- Non-addictive with an instant osmotic action.
Evidence-Based Design
Developed through rigorous scientific research, including clinical trials and studies, ALLERSPRAY-G's efficacy and safety are well-documented. Its barrier-forming properties and resistance to mechanical pressures make it a reliable choice for allergic rhinitis treatment.
Clinical Efficacy
A randomized, double-blind clinical trial highlighted its effectiveness in reducing nasal congestion and irritation, leading to a noticeable improvement in quality of life. The trial also showed significant reductions in total nasal and ocular symptoms within weeks.
TYPE OF TRIAL: Randomized, double-blind clinical trial against placebo | 3 weeks
PATIENTS: N=51 | Test product = 33 ; Comparator = 15
INCLUSION: Positive skin prick test for allergens and positive basophil count (>10 eosinophils/hpf)
Main results :
TOTAL NASAL SYMPTOMS
- 38 % reduction in total nasal symptoms within 1 week
- 58 % reduction in total nasal symptoms within 2 weeks
- 73 % reduction in total nasal symptoms within 3 weeks
TOTAL OCULAR SYMPTOMS
- 38 % reduction in total ocular symptoms within 1 week
- 55 % reduction in total ocular symptoms within 2 weeks
- 64 % reduction in total ocular symptoms within 3 weeks
OTHER PARAMETERS
- 50 % improvement in RQLQ score (Rhinoconjunctivitis Quality of Life Questionnaire)
- Rescue medicine score : 80 % for placebo group vs 29 % for test group
- Reduction in eosinophil count : 15 % for placebo group vs 68 % for test group
- 68 % reduction of allergic activity within 3 week
Product Details
- Indication: Treatment of mild to moderate intermittent seasonal allergic rhinitis
- Composition: Includes glycerol, water, and extracts from Camellia sinensis, Curcuma longa, Panax ginseng, Urtica dioica, with EO of Citrus limonum.
- Presentation: 15ml nasal spray, approximately 125 sprays.
- Class: Transitioning from MDD Class I to MDR Class IIa certification.
- Usage: 2 sprays in each nostril, 2-3 times a day.
- Precautions: Topical use only. Keep out of reach of children.
- Age: Suitable for adults 18 and over.
- Storage: Store below 25°C.
- Manufacturer: VITROBIO, France.
- Product Code: AAG.