Patient's Benefits
- Reduction of nasal symptoms such as nasal congestion, sneezing, nasal itching and rhinorrhea within 6 days
- Reduced nasal congestion from day 1 of treatment
- Reduced eye symptoms such as redness, itching and tearing within 6 days
- Improvement in quality of life from the reduction of both nasal and ocular symptoms
- Reduced need for allergic rhinitis medication
- Improved patient well-being and sleep quality
- Contributes to the reduction of respiratory symptoms
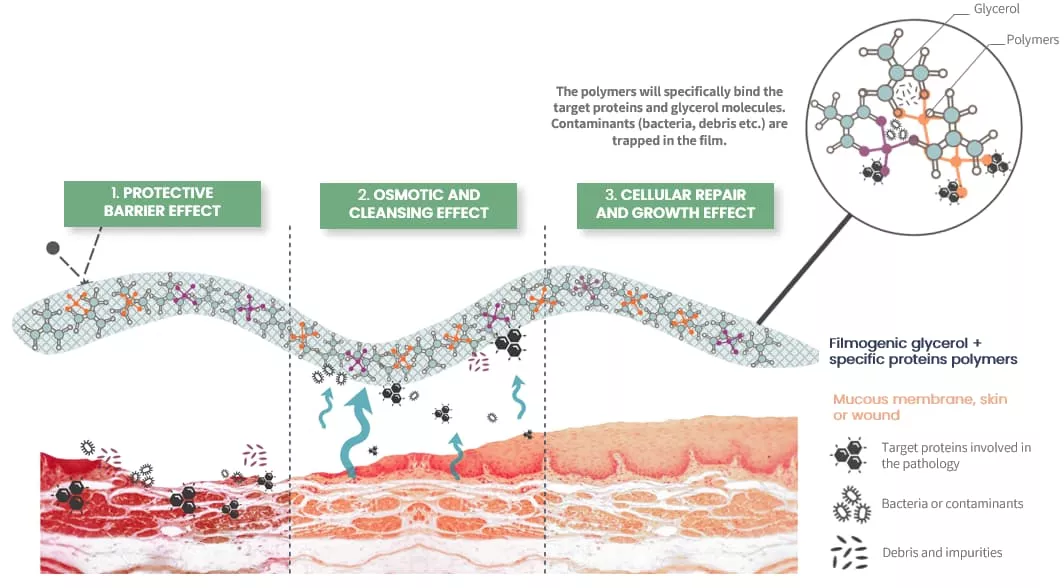
Mode of action
ALLERPUR stands out with its formula that not only provides quick relief but also offers a long-term solution to allergic rhinitis caused by airborne environmental irritants.
- Protective Barrier: It forms a thin, protective film over the mucosa, creating a mechanical barrier that shields it from direct contact with airborne environmental irritants.
- Mechanical Cleaning: Utilizes osmotic action to attracts, traps, and removes contaminants, helping to reduce inflammation and alleviate symptoms.
- Cellular Repair: Promotes the healing and regeneration of the nasal mucosa, restoring its natural barrier against future allergic triggers.
Clinical Trial
A randomized, double-blind clinical trial (n = 49) confirmed the efficacy of the product in relieving nasal and ocular allergy symptoms over 3 weeks.
The treatment led to a 73% reduction in total nasal symptoms (congestion, rhinorrhea, sneezing, itching) and a 60% reduction in ocular symptoms (redness, tearing, itching) by day 22. Respiratory symptoms (cough, wheezing, chest tightness) also improved by 41%.
A 46% improvement in quality of life was recorded (RQLQ score), and the need for rescue medication was significantly lower in the test group (3.4% vs 23.5% in the placebo group).
These results demonstrate the product’s strong potential to relieve allergic symptoms and improve daily well-being, with excellent tolerability.
Product Details
- Indication: Treatment of mild-to-moderate, intermittent, allergic rhinitis caused by environmental irritants.
- Composition: Made with ingredients of natural origin
- Presentation: Nasal spray 15ml.
- Class: MDR Class IIa.
- Age: Suitable for adults 18 and over.
- Storage conditions: Below 25°C.
- Legal manufacturer: VITROBIO (France).
- Product code: PC.
Need more information
 This image is a representation. Your design will be featured in the final product.
This image is a representation. Your design will be featured in the final product.