VITROBIO
Innovative
Medical Devices
Branded turnkey solutions meeting the requirements of MDR 2017-745
Turnkey medical devices under your brand

Vitrobio Achieves MDR 2017/745 Certification
We are proud to announce that Vitrobio has obtained the MDR 2017/745 certification for our range of spray medical devices.
This certification, achieved after three years of rigorous effort, ensures our products meet the latest European safety and quality standards, reflecting our commitment to excellence in the medical device industry.
15 therapeutic indications

Research & Development
Our research efforts commence with foundational studies conducted in our laboratory or in collaboration with INSERM. Subsequently, we progress to clinical trials and the advancement of our groundbreaking technologies.

Quality &
Regulatory
As the legal manufacturer, we compile all the necessary regulatory documentation for the products' market approval, with GMED acting as our notified body.
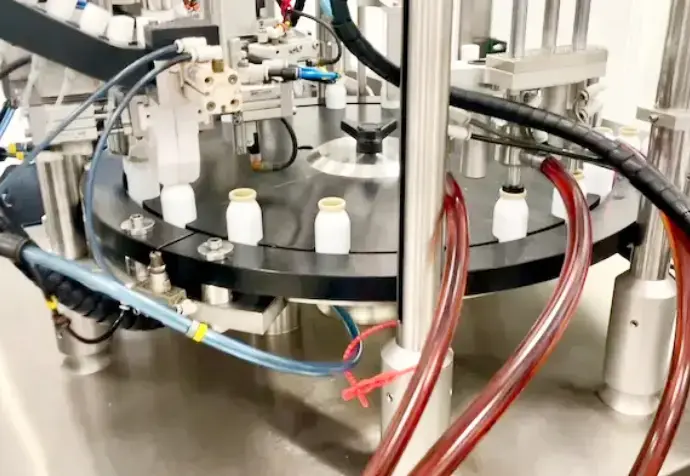
Production & Logistics
We produce all our medical devices in-house through our production lines. Our internal production capacity allows us to manufacture up to 20K units per day and can be expanded with the help of our subcontractors.
Patented technology
Our products are based on innovative polymer technology. This technology is protected worldwide by our patents.
Find out more about our technology.
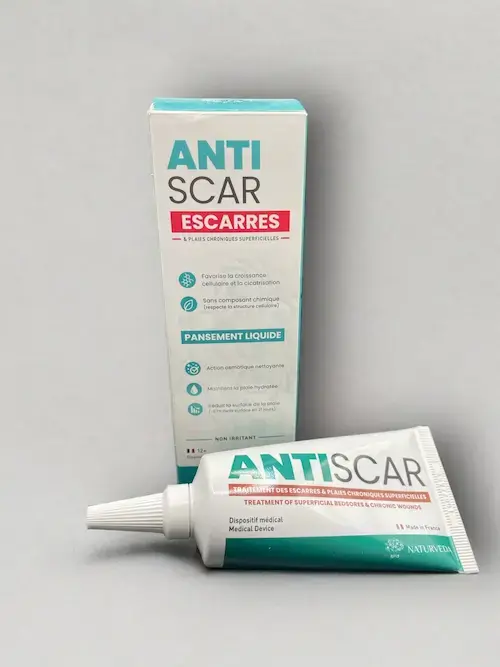
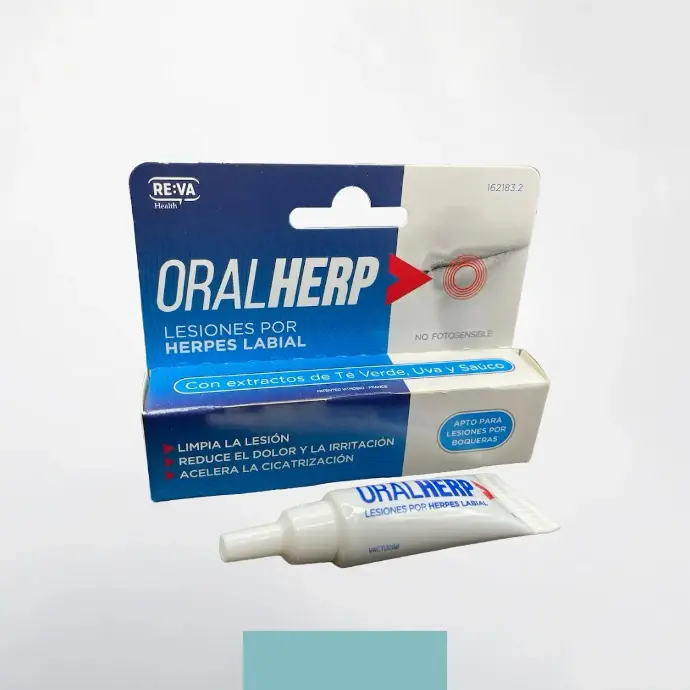
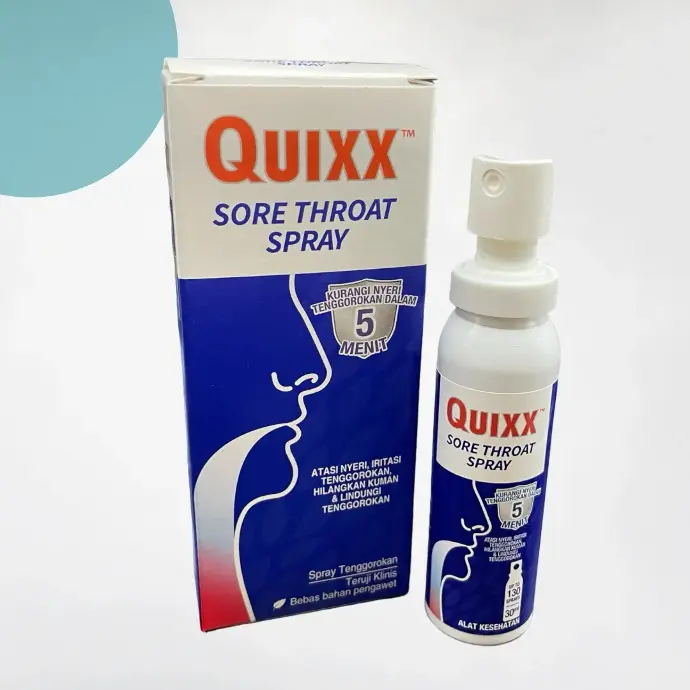
More than 15 pathologies
We have around 30 formulations for 15 different indications. From colds and migraines to coughs, allergic rhinitis and bedsores, we're sure to have a solution to meet your needs.
ISO 13485
As a manufacturer of medical devices, we are accredited to ISO 13485, which sets out the requirements for a quality management system.
See our ISO 13485 certificate
MDR 2017-745
Our notified body is GMED. We are proud to announce that Vitrobio has obtained the MDR 2017/745 certification for our range of spray medical devices.
Click here to find out more.
Some of our references
They trust us for their medical devices






Do you want to expand your product range?
Don't hesitate to contact us to discuss your project. Our team will be happy to answer any questions you may have.