2017/745 Regulation (MDR) – Vitrobio Certification Update
As we informed you in our July newsletter:
Vitrobio is already committed to the transition to MDR. An application for certification under Annex XI part A of Regulation 2017/745 has been submitted to the GMED, for the entire product portfolio. Conformity assessment services are currently underway.
Vitrobio was audited in March 2023 by the GMED (Initial certification audit under the Regulation 2017/745). This on-site audit did not reveal any major non-compliance (0 major non-conformities). In parallel, the technical documentation for Vitrobio products is currently being assessed. Certification is conditioned by the conclusions of both on-site audit and documentary review. At this stage, we hope to obtain certification in mid-2024.
Vitrobio products benefit from the extended transition period. They can therefore be produced under certification in accordance with Directive 93/42/EEC (MDD) until 31/12/2028 and can then be kept on the market until they expire.
One certification does not cancel the other, so the same product can be manufactured under both directive and regulation certification at the same time.
Please note that HG-VB, indicated for the treatment of genital herpes, and formula VB-PHR-E, indicated for the treatment of sore throat in children, do not benefit from the transition period and will therefore no longer be manufactured after 26 May 2024.
To date, Vitrobio’s certification process is proceeding according to the deadlines previously announced.
2017/745 Regulation (MDR) – UDI information
Vitrobio, like all legal manufacturers of medical devices in Europe, must comply with the requirements relating to the UDI identification of these products. We are committed to effective collaboration with our partners. So, we provide you with the information that may impact your business and we will support you in the implementation of changes.
Regulatory requirements imposed on all manufacturers: From 26 May 2023, all Class IIa medical devices manufactured in accordance with the requirements of Regulation 2017/745 must bear the unique UDI identification number.
This is made up of the UDI-DI and the UDI-PI as shown in the illustration below:

Affixing the UDI to legacy devices manufactured under MDD certification remains optional.
Applying the UDI (in human-readable format only) to the device itself (on the bottle) will not be compulsory until 26 May 2025.
The solutions implemented by Vitrobio: All Vitrobio products will be concerned as soon as they are manufactured under MDR certification.
The UDIs for Vitrobio products will therefore consist of:
- the UDI-DI composed of the GTIN number generated by Vitrobio (legal manufacturer) and preceded by the tag (01)
- the UDI-PI composed of the batch number preceded by the tag (10) and the date of manufacture preceded by the tag (17).
The UDI number will appear in data matrix format and in human-readable format on the secondary packaging of products manufactured by Vitrobio under MDR certification.
The UDI number will appear in human-readable format on the primary packaging of products manufactured by Vitrobio under MDR certification from 26 may 2025.
2017/745 Regulation (MDR) – UDI impact on your packaging
SECONDARY PACKAGING (BOXES): Prior to any manufacture of devices under MDR certification, it will be necessary to revalidate boxes, labels and leaflets in order to comply with the requirements of regulation 2017/745. With regard to the UDI, each of the boxes will have to include a white, unvarnished, unembossed reserve area on the flap below the box as shown in the illustration below:
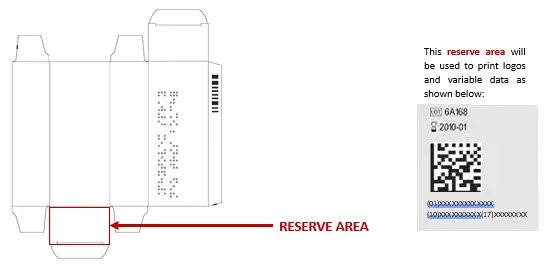
PRIMARY PACKAGING (SPRAYS OR TUBES): The UDI will be printed on the primary packaging of products manufactured under MDR certification from 26 May 2025, in human-readable format only: (01)XXXXXXXXXXXXX(10)XXXXXXXXXX(17)XXXXXXXX
- Sprays: Vitrobio will do its upmost to ensure that the technical solution for printing the UDI does not require defining a reserve zone on the label. The UDI marking is envisaged directly on the bottom of the bottle.
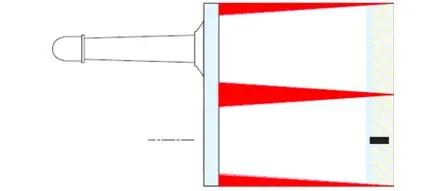
- Tubes: The UDI printing for tubes will require to define a reserve zone on the label. Depending on the labelling as well as the size of the tube the reserve zone will be treated individually.
Example of a reserve zone represented in red on the label for a 10mL tube:
Sustainability – FSC paper for boxes and leaflets
Respecting new regulations in Europe for more environmentally sustainable and circular products, Vitrobio works to improve the products that are distributing in the sense of eco-design. For that, in April 2024, it will be possible, on demand, to get secondary packing made of FSC materials. The cost of articles made of cardboard is slightly impacted and Vitrobio is working with qualified suppliers to reduce at the maximum the incidence of this change.

Example of FSC logo applied on the cardboard box surface in France
Nasal spray manufacturing – New pumps and containers
From May 2024, nose sprays will be manufactured with pumps and bottles slightly modified without affecting current volumes & functioning. Before implementing this change, Vitrobio will have ensured that there is no impact on the safety, stability and performance of the products. This change has no impact on the devices’ usability. Vitrobio will make available to its distributors all documentation relating to this change and will assist its partners in their submissions to their national authorities if necessary.
You can be assured of Vitrobio’s support in implementing these changes.
We remind our distributors, who place our products on the market outside the European Union, that it is their responsibility to notify changes to the national authorities of the countries concerned.