Patient's Benefits
- Provides relief from throat pain from the first application
- Reduces throat redness from the first application
- Diminishes throat irritation within the first application
- Relieves swelling upon first application
- Achieves a 50% reduction in symptoms within 2 days of treatment
- Enhances overall well-being
- Reduces the need for antibiotic use
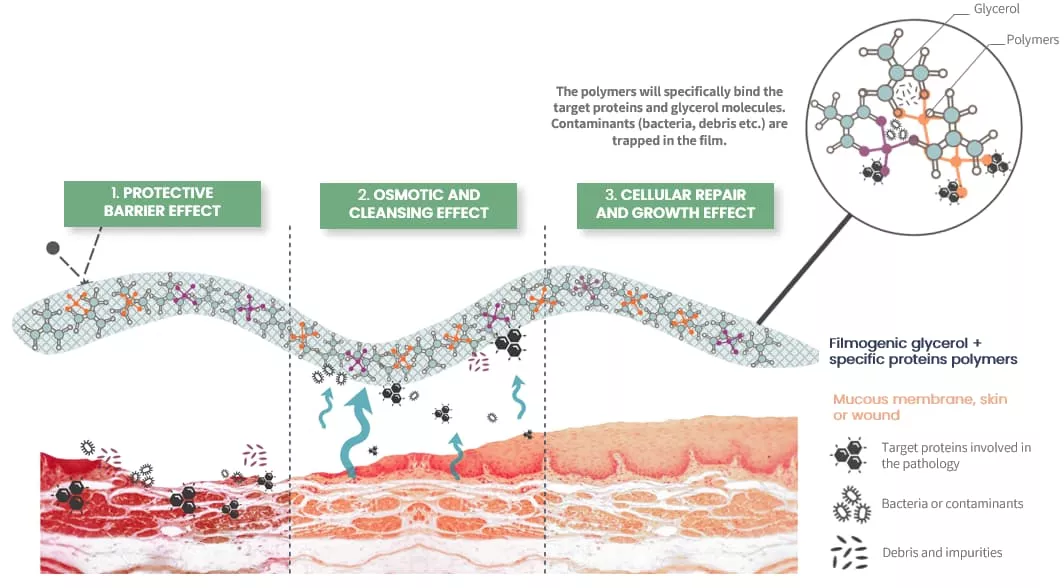
Mode of Action
- Protective Barrier: It forms a thin, protective film over the mucosa, creating a mechanical barrier that shields it from direct contact with irritants.
- Mechanical Cleaning: Uses osmotic activity to draw excess water from swollen throat tissues, helping to reduce swelling, eliminate contaminants, and alleviate inflammation and related symptoms.
- Cellular Repair: Promotes healing and regeneration of the mucosa for sustained relief.
Clinical study
An open-label, single-arm clinical trial (n = 60) demonstrated the product’s effectiveness in relieving throat symptoms and improving quality of life over 10 days.
Throat pain, irritation, and redness were significantly reduced within 2 hours of the first application. Bacterial count dropped by 81% within 7 days, reaching normal levels by day 10. Recovery was rapid, with 95% of patients fully recovered by day 10. The need for antibiotics was also strongly reduced, confirming the product’s efficacy and potential to limit antibiotic use.
Product Details
- Indication: Used in adults for the symptomatic treatment of mild to moerate acute pharyngitis, bacterial or viral.
- Composition: Made from natural origin ingredients
- Presentation: 30ml throat spray.
- Class: MDR Class IIa.
- Age: Suitable for adults 18 and over.
- Storage conditions: Below 25°C.
- Legal manufacturer: VITROBIO (France).
- Product code: PHN.
Need more information
 This image is a representation. Your design will be featured in the final product.
This image is a representation. Your design will be featured in the final product.