Patient's benefits
- Reduction in rhinorrhoea within 6 days.
- Improvement in nasal congestion within the first 30 minutes.
- Reduction in headaches from the third day.
- Reduction in facial pain from the third day.
- Reduced use of antibiotics.
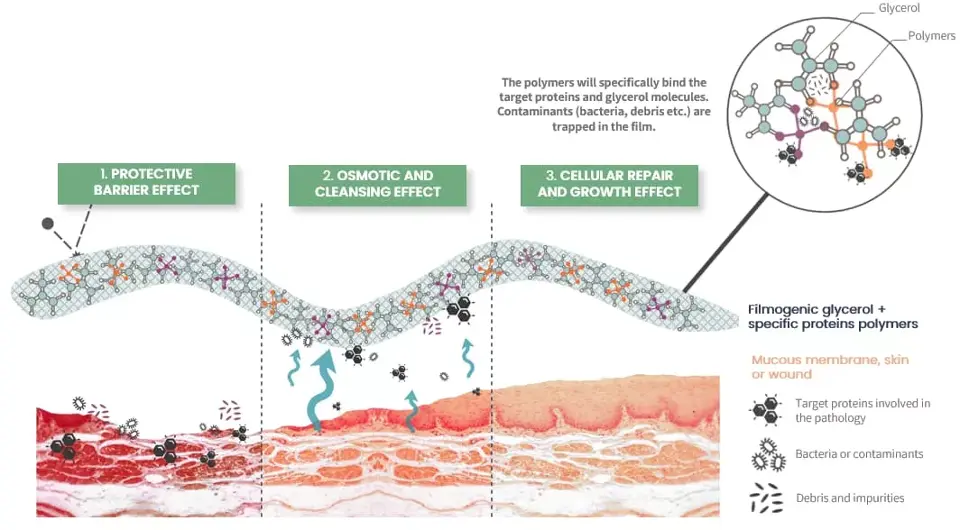
Mode of action
- Protective Barrier: It forms a thin, protective film over the mucosa, creating a mechanical barrier that shields it from direct contact with external agressors such as pathogens and environmental factors that can prolong or exacerbate rhinosinutis.
- Mechanical Cleaning: Utilizes osmotic activity to draw out fluids and irritants, cleansing the nasal mucosa, reducing swelling, and alleviating nasal congestion, indirectly addressing inflammation.
- Cellular Repair: Promotes healing and regeneration of the mucosa for sustained relief.
Clinical Trial
NESOSPRAY HE has proven effective in treating rhinosinusitis symptoms in a randomized, double-blind, placebo-controlled clinical trial (n = 54).
The spray led to a 58% reduction in nasal congestion within 6 days, with effects starting as early as 30 minutes post-application. Rhinorrhea initially increased due to enhanced secretion clearance, but then decreased by 77% at day 14. Patients also reported rapid improvements in headache (−64%), facial pain (−66%), and postnasal drip (−26%) within 3 days. Only 3% of patients in the NESOSPRAY group required rescue antibiotics vs 19% in the placebo group. The treatment was associated with a marked improvement in quality of life, confirming its efficacy and safety for rhinosinusitis relief.
Product Details
- Indication: Used in adults for the treatment of acute rhinitis, rhinosinusitis and rhinopharyngitis.
- Composition: Made from vegetal origin ingredients
- Presentation: Nasal spray 15ml.
- Class: MDR Class IIa.
- Age: 18+.
- Storage conditions: Below 25°C.
- Legal manufacturer: VITROBIO (France).
- Product code: NHE.
Need more information
 This image is a representation. Your design will be featured in the final product.
This image is a representation. Your design will be featured in the final product.