Patient's benefits
- Offers gradual relief from difficulty swallowing
- Begins to soothe throat irritation with the first use.
- Reduces throat swelling progressively.
- Reduces throat redness.
-
Contributes to reducing whitish deposits on the throat from the first application.
- Enhances overall well-being.
- Reduces the need for antibiotic use.
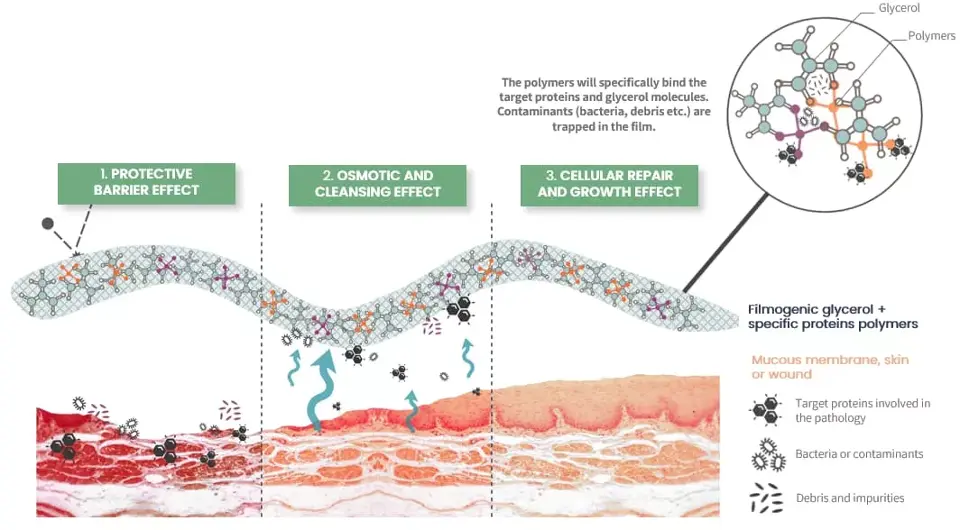
Mode of Action
- Protective Barrier: It forms a thin, protective film over the mucosa, creating a mechanical barrier that shields it from direct contact with irritants.
- Mechanical Cleaning: Uses osmotic activity to draw excess water from swollen throat tissues, helping to reduce swelling, eliminate contaminants, and alleviate inflammation and related symptoms.
- Cellular Repair: Promotes healing and regeneration of the mucosa for sustained relief.
Clinical study
A randomized, double-blind clinical trial confirmed the effectiveness of the product in relieving throat symptoms in 30 patients.
Significant improvements were observed in difficulty swallowing and throat swelling by day 7, with visible reductions from the first application. Irritation, redness, and white throat deposits also decreased markedly, with notable improvements by day 3 and up to 80% reduction by the end of treatment. The need for antibiotics was significantly lower in the test group compared to placebo, confirming both the efficacy and potential to reduce antibiotic use.
Product Details
- Indication: Used for the symptomatic treatment of acute pharyngitis, bacterial or viral, in children aged three (3) years and older.
- Composition: Made from natural-origin ingredients
- Presentation: 20ml Throat spray.
- Class: MDR Class IIa.
- Age: Suitable for children aged 3 years and older.
- Storage conditions: Below 25°C.
- Legal manufacturer: VITROBIO (France).
- Product code: PEN.
Need more information
 This image is a representation. Your design will be featured in the final product.
This image is a representation. Your design will be featured in the final product.