Patient's benefits
- Protective barrier effect against allergens
- Reduction of nasal symptoms such as nasal congestion, sneezing, nasal irritation andrhinorrhea within 7 days
- Reduced eye symptoms such as redness, tearing and burning within 7 days
- Improvement in quality of life from the reduction of both nasal and ocular symptoms
- Reduced need for allergic rhinitis medication medication
- Prevent secondary infection and progression to a severe, chronic form
- Improved patient well-being and sleep quality
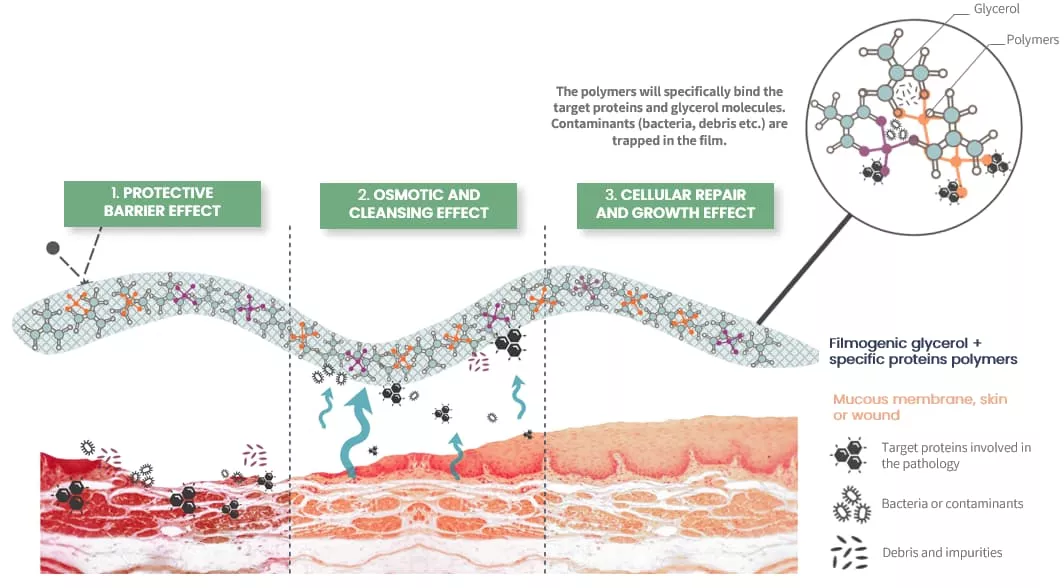
Mode of Action
- Protective Barrier: Establishes a filmogenic shield on the nasal mucosa, preventing irritants from triggering allergic reactions.
- Mechanical Cleaning: Utilizes osmotic activity to remove contaminants, reducing inflammation and symptoms.
- Cellular Repair: Promotes healing and regeneration of the nasal mucosa for sustained relief.
Clinical study
A randomized, double-blind clinical trial (n = 51) confirmed the effectiveness of the product in relieving allergic rhinitis symptoms over 3 weeks.
It led to a 73% reduction in total nasal symptoms and a 64% reduction in ocular symptoms, with improvements starting from the first week. The RQLQ score improved by 50%, indicating a clear enhancement in quality of life.
The need for rescue medication was significantly lower in the test group (29% vs 80%), and along with a marked reduction in eosinophil count.
These results highlight the product’s anti-allergic effect and excellent clinical benefit in managing nasal and ocular allergy symptoms.
Product Details
- Indication: Used in adults for the symptomatic treatment of seasonal and intermitent mild-to-moderate allergic rhinitis.
- Composition: Made from plant-based ingredients
- Presentation: 15ml nasal spray
- Class: MDR Class IIa.
- Age: Suitable for adults 18 and over.
- Storage: Store below 25°C.
- Manufacturer: VITROBIO, France.
- Product Code: AAG.
 This image is a representation. Your design will be featured in the final product.
This image is a representation. Your design will be featured in the final product.