Patient's benefits
- Reduces associated symptoms such as tingling, burning and itching.
- Relief pain
- Reduces swelling, soreness and tenderness
- Limits viral spread
- Promotes and accelerates lesion healing
- Reduces blister oozing
- Cleans the affected area from contaminants, cellular debris and irritants
- Protects the affected area from secondary infection and irritants
- Improves patient well being
Mode of Action
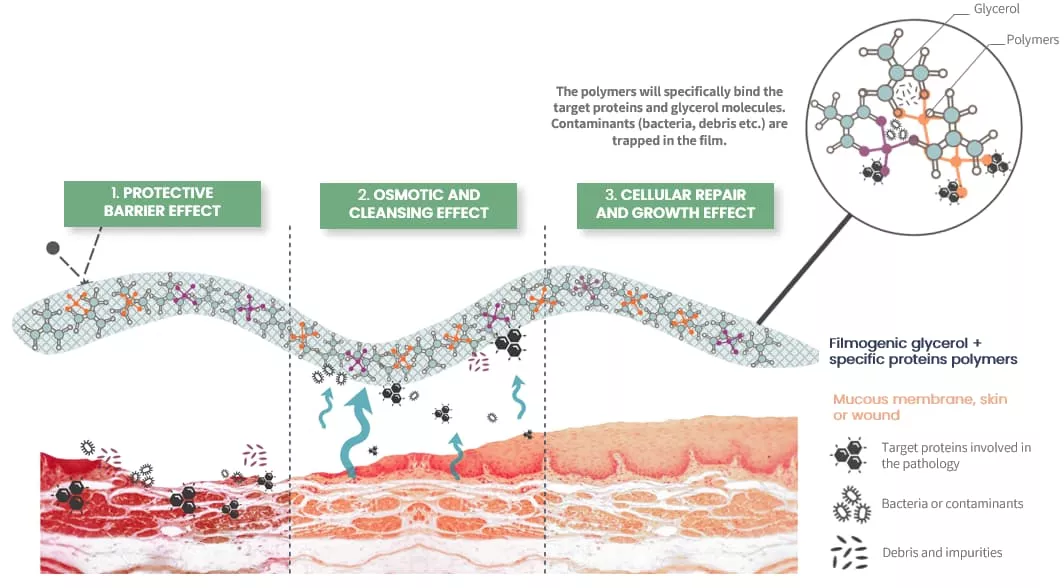
- Protective Barrier: Forms a protective film on the affected area, shielding it from external contaminants and reducing further irritation. This creates a favorable environment for improved comfort while keeping the lesion hydrated, thanks to the presence of glycerol.
- Mechanical Cleaning: Utilizes osmotic action to keep the affected area clean by drawing out excess fluid and removing contaminants, helping to reduce inflammation, swelling, and symptoms.
- Cellular Repair: Promotes and accelerates healing and regeneration of the affected area, providing sustained relief while maintaining a favorable environment for improved comfort and supporting the skin's natural healing process.
Clinical study
ORALHERP has demonstrated strong clinical efficacy and safety in managing labial herpes. In a 14-day open-label, single-arm, multicenter trial involving 60 patients, the treatment led to a rapid reduction in viral load (−38% at 2 hours, reaching 100% by day 14) and progressive lesion healing (−55% by day 4, −87% by day 7, full resolution by day 14). Patients also experienced early relief of pain, burning, itching, and tingling, with symptoms significantly reduced by day 4 and completely resolved by day 14. Blister oozing and adjacent lesions also improved markedly. The product was very well tolerated, with no adverse effects and no impact on laboratory safety parameters. These findings support ORALHERP as an effective and safe topical treatment for labial herpes, providing both symptom relief and accelerated healing.
Product Details
- Indication: Used for the treatment of labial herpes.
- Presentation: Tube 6ml.
- Class: MDR Class IIa.
- Age: Suitable for adults 18 and over.
- Storage conditions: Below 25°C.
- Legal manufacturer: VITROBIO (France).
- Product code: HL.
Need more information
 This image is a representation. Your design will be featured in the final product.
This image is a representation. Your design will be featured in the final product.