Patient's benefits
- Reduces mucositis severity
- Immediate and sustained pain relief
- Burning sensation alleviation
- Improved oral hydration
- Helps reduce the formation and size of ulcers
- Improved quality of life
- Helps enhance nutrition
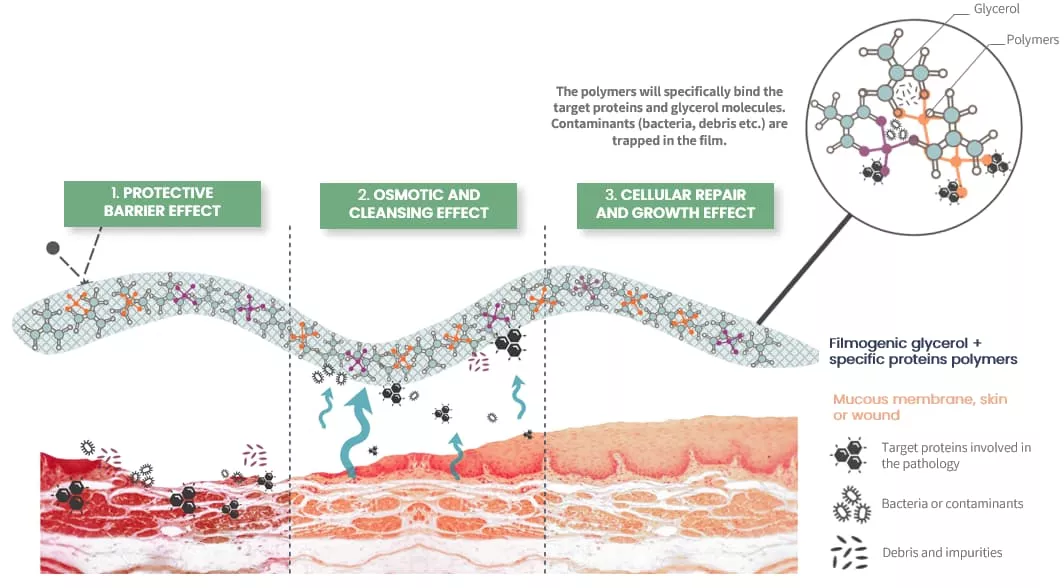
Mode of Action
- Mechanical Cleaning: The product works to cleanse the oral cavity, removing impurities and contaminants that can exacerbate inflammation and delay healing.
- Protective Barrier: OROSOL creates a protective barrier in the oral cavity, shielding the mucous membranes from further damage and allowing the healing process to begin.
- Cellular Repair: OROSOL promotes the repair and growth of cells in the oral cavity, accelerating the healing process and reducing the duration of symptoms.
Clinical Trial
OROSOL® has shown strong clinical effectiveness in the management of oral mucositis. In a 28-day clinical trial, patients using OROSOL® experienced a 70% reduction in mucositis severity, compared to just 10% in the control group. There was also a significant decrease in pain (from 7.19 to 2.05) and burning sensation (from 7.25 to 1.92). OROSOL® also reduced infection risk, minimized new ulcer formation, and improved eating ability and oral hydration. The treatment was well tolerated, with only mild and temporary sensations after application. These results support OROSOL® as an effective and safe therapeutic option for improving quality of life in patients with oral mucositis.
Product Details
- Indication: Used in adults and children aged eight years and older, for the treatment of oral mucositis induced by chemotherapy or radiotherapy.
- Composition: Made from natural-origin ingredients
- Presentation: Oral spray 20ml.
- Class: MDR Class IIa.
- Age: Suitable for adults and children aged eight years and older (8+).
- Storage conditions: Below 25°C.
- Legal manufacturer: VITROBIO (France).
- Product code: OS.
Need more information
 This image is a representation. Your design will be featured in the final product.
This image is a representation. Your design will be featured in the final product.